Abstract
Background. Failure after anti-CD19 Chimeric Antigen Receptor (CAR) T-cells is associated with poor prognosis. Several reports showed 65.5 to 83% of CAR T-cell failures occur in the first 3 months (Early Failure, EF), while late failures (LF), after 3 months, represented only 12.5 to 34.5 %. Longer duration of response to CAR T-cell therapy has been associated with better survival in a real-life setting.
Here, we analyzed clinical features, patterns of treatment and outcomes of LF patients (pts) from DESCAR-T, a nationwide registry collecting real-life data for pts treated with approved CAR T-cell therapy in France.
Patients and Methods. The present analysis (April 29, 2022) included 977 pts with Relapsed/Refractory (R/R) aggressive B-cell lymphoma (BCL) registered in DESCAR-T from July 2018 and infused with either axicabtagene ciloleucel (axi-cel; n=611) or tisagenlecleucel (tisa-cel; n=365) (infusion data missing for one pt). All pts were informed before being included in DESCAR-T registry. Progression and relapse after CAR T-cells were based on the Cheson 2014 response assessment criteria.
Results. Overall, 431/977 pts (44.1%) failed (Progressive Disease or Relapse) after CAR T-cell infusion, of whom 145 (33.6%) were LF. For LF patients, histological subtypes were diffuse large B-cell lymphoma (DLBCL, n=109, 75.2%), primary mediastinal B-cell lymphoma (PMBL, n=6, 4.1%), high-grade B-cell lymphoma (HGBCL, n=6, 4.1%), transformed follicular lymphoma (t-FL, n=15, 10.3%), or other (n=9, 6.2%: primary central nervous system lymphoma [PCNSL, n=1]; transformed marginal zone lymphoma [t-MZL, n=5]; grey zone lymphoma [GZL, n=2]; Richter syndrome, n=1). At time of CAR T-cell eligibility, median age was 62 years (range 19-77), 40% of pts were aged >65 yrs, and 58.6% were male; 120 (85.1%) had advanced disease (stage III/IV) and 78 (57.8%) had an age-adjusted International Prognostic Index (aaIPI) of 2-3. The median number of treatment lines prior eligibility was 3 (range 2-10). Thirty-six (24.8%) pts were transplant recipients (34 auto-HSCT and 2 allo-HSCT). At time of CAR T-cell infusion, 12 pts (8.4%) had ECOG PS >2, 72 (50.7%) elevated LDH level and 34 (23.4%) bulky disease. Median ferritin and C-reactive protein blood levels were 474.5 µg/l and 8 mg/dl respectively. Bridging therapy was administered to 82.8% of pts (chemotherapy-based for 76.7% of pts). Overall Response Rate (ORR) to CAR T-cell therapy among LF pts was 90.3%, among whom 76 (52.4%) were in complete metabolic response (CMR); 14 pts were enlisted as non-responders in the registry despite not showing clear signs of progression at 3 months. Median time from infusion to failure was 4.11 months (3 - 21.5, IQR 3.1). After failure, 104 LF pts (71.7%) received a systemic treatment as follows: 41 (33.1%) IMiDs-based, 22 (17.7%) chemotherapy-based, 18 (14.5%) bi-specific antibodies (BsAb), 16 (12.9%) targeted therapy (BTKi, MALT-1 inhibitors), 7 (5.6%) anti-PD1. Eighteen (14.5%) pts received radiotherapy because expressed localized disease, while 20 (13.8%) were not treated. The ORR after post CAR T-cell failure treatment was 15.4%, with 10.6% of CMR. At a median follow-up from first progression of 15 months (95% CI, 12-17), LF pts had a median PFS of 4.2 months (95% CI, 3.4-6) after treatment for CAR T failure, and a median overall survival (OS) of 12.1 months (95% CI, 6.9-15.7).
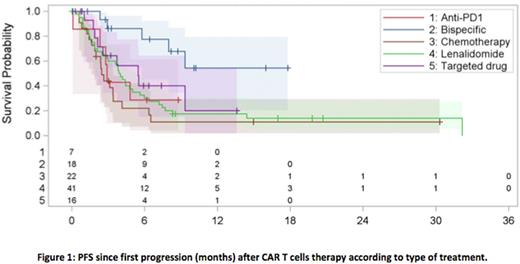
Between therapy groups, when compared to chemotherapy [6-months PFS and OS of 22% (95% CI, 7.1-42) and 39.4% (95% CI, 18.3-60.1), respectively], only pts treated with BsAb showed a benefit in terms of 6-months PFS [77.5%, HR=0.188 (95% CI, 0.069-0.509), P=0.001] (Figure 1), while 6-months OS was longer in both targeted therapy [76.6%, HR=0.278 (95% CI, 0.093-0.83), P=0.02] and BsAb groups [92.9%, HR=0.167 (95% CI, 0.049-0.572), P=0.004]). For pts treated with radiotherapy, 6-months PFS and OS were 64.2% (95% CI, 36.9-82.1) and 94.1% (95% CI, 65-99.1), respectively.
Conclusion. This is the first analysis of pts with R/R aggressive BCL that failed after 3 months from CAR T-cell therapy. Even though the groups were small in size, the best PFS and OS were observed in the BsAb treatment group. Radiotherapy can obtain prolonged responses in selected pts with localized disease. Different biological mechanisms of relapse and sensitivity to adoptive immunotherapy could explain the later loss of response in LF subgroup of pts and should be investigated.
Disclosures
Bachy:Kite, Gilead, Novartis, Roche, Incyte, Miltenyi Biotech, Takeda, Sanofi: Honoraria; Roche, Gilead, ADC Therapeutics, Takeda, Novartis, Incyte: Membership on an entity's Board of Directors or advisory committees; Amgen, BMS: Research Funding; Hospices Civils de Lyon: Current Employment. Cartron:Gilead, Novartis, Mylteni, Sanofi, Abbvie, Takeda, Roche, Janssen, Celgene, Novartis, Bristol Myers Squibb: Honoraria; MabQi, Ownards Therapeutics, Abbvie, Roche, Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Morschhauser:Roche, Chugai: Other: Scientific lectures; gilead, roche: Consultancy; Gilead, Novartis, BMS, épizyme, miltenyi, Abbvie, genmab, Roche, AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Tournilhac:Janssen: Honoraria, Other: Travel grant , Research Funding; Takeda: Honoraria, Other: Travel grant , Research Funding; Abbvie: Honoraria, Other: Travel grant , Research Funding; Gilead: Honoraria, Other: Travel grant , Research Funding; Securabio: Honoraria, Other: Travel grant , Research Funding; IdeoGen: Honoraria, Other: Travel grant , Research Funding. Sylvain:Abbvie, AstraZeneca, Atara, Novartis, Gilead/Kite, Takeda: Consultancy. Gros:Milteny: Honoraria; Novartis: Honoraria; BMS: Honoraria; Kite/Gilead: Honoraria. Casasnovas:Roche, Takeda, BMS, MSD, Gilead/Kite, Janssen, ADC Therapeutics, Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche, Gilead, Takeda: Research Funding; Roche, Takeda, Merck, BMS, Gilead/Kite, Abbvie, ADC therapeutics, INCYTE, AstraZeneca: Honoraria. Bories:Novartis, Kite/Gilead, BMS-Celgene, Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Mohty:Celgene: Honoraria; Jazz Pharmaceuticals: Honoraria, Research Funding; Takeda: Honoraria; Bristol Myers Squibb: Honoraria; Amgen: Honoraria; Astellas: Honoraria; Novartis: Honoraria; GSK: Honoraria; Adaptive Biotechnologies: Honoraria; Oncopeptides: Honoraria; Pfizer,: Honoraria; Sanofi: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Gilead: Honoraria. Carre:Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees. Le Gouill:Novartis, Kite/Gilead, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support. Houot:Bristol-Myers Squibb, Celgene, Gilead Sciences, Incyte, Janssen, Kite, MSD, Novartis and Roche: Honoraria. Di Blasi:Novartis, Kite/Gilead, Janssen, Pfizer, Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal